PROMPT
Prevention of early mortality by presumptive tuberculosis treatment in HIV infected patients initiating antiretroviral therapy
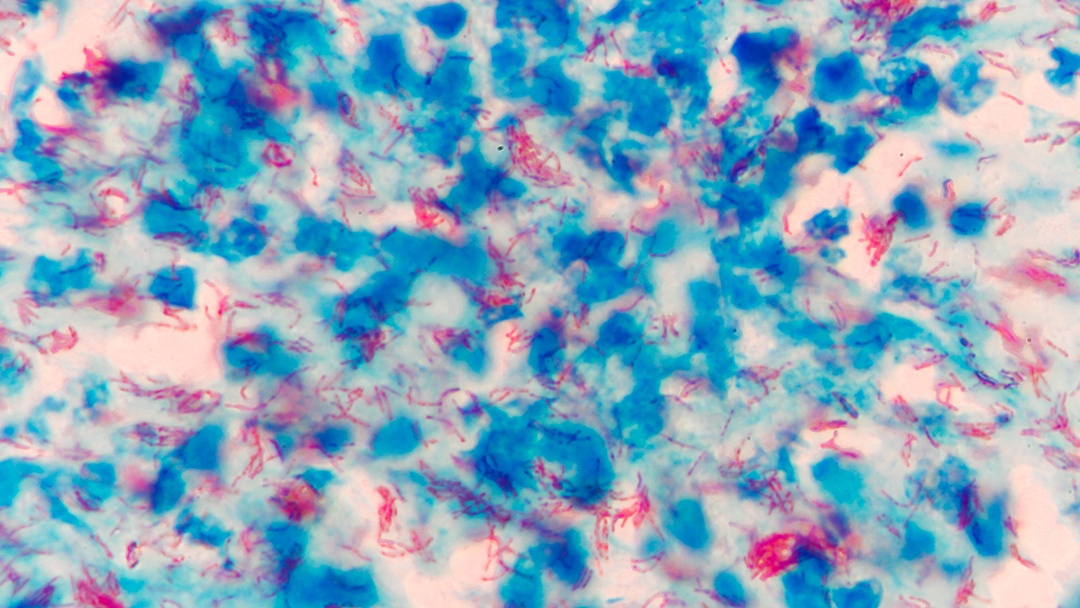

Objective
The overall goal of the project is to evaluate a strategy for reducing early mortality during antiretroviral treatment in settings with high incidence of TB and limited facilities for diagnosing TB in symptomatic, severely immunosuppressed HIV-infected patients. We also aim to identify the patients who would most benefit from this intervention.
Description
Despite the large-scale roll out of antiretroviral treatment (ART), which has great benefit long-term, there is substantial mortality of HIV-infected patients waiting for ART initiation during the first months of treatment. With this project we aim to improve early survival of patients on ART by early and presumptive treatment of tuberculosis (TB).
The primary objective of this project is to test, in a randomized-controlled trial, the hypothesis that in symptomatic HIV infected patients with severe immune suppression, presumptive treatment with a full course of anti-TB drugs reduces mortality in the first six months after antiretroviral treatment initiation. We hypothesize that, in TB endemic settings, a large proportion of symptomatic, severely immune suppressed patients have disease due to actively multiplying M. tuberculosis. Therefore presumptive anti-TB treatment, in patients without a well-established diagnosis of TB, may substantially reduce mortality when combined with ART. If proven to be efficacious, presumptive TB treatment would be an affordable intervention that can be readily implemented in most settings in sub-Saharan Africa where patients with late-stage HIV disease are diagnosed and treated.
The entire project will also be used to build or strengthen capacity in four sites throughout sub-Saharan Africa for clinical trials of therapeutic interventions of HIV and/or TB disease.
Partners
Infectious Disease Institute (IDI) and the Makerere University College of Health Sciences (MUCHS) in Kampala, Uganda
University of Limpopo (UL) in Limpopo, South Africa
Medical Research Unit of the Albert Schweitzer Hospital (HAS-MRU) in Lambaréné, Gabon
Catholic University of Mozambique and the Center for Infectious Disease Research (UCM-CIDI) in Beira, Mozambique.
Institute of Tropical Medicine and the University of Antwerp
Institute of Tropical Medicine of the University of Tuebingen (ITM-UT)
Funders
EDCTP
Countries
Mozambique
Uganda
Gabon
South Africa
